Originally published in ProPublica
ProPublica is a Pulitzer Prize-winning investigative newsroom. Sign up for The Big Story newsletter to receive stories like this one in your inbox.
The drug Plaquenil keeps Anna Valdez’s lupus in check.
Late last week, as she sheltered in place at her home outside Santa Rosa, California, Valdez called her local pharmacy and ordered a refill to treat her autoimmune disorder, thinking a 90-day supply would help her ride out the coronavirus outbreak.
But the pharmacy told her it had only 10 pills left. Valdez called other pharmacies. They, too, had run out.
Valdez and lupus patients around the country have learned in recent days that an extraordinary force has upended the supply chain they all rely on: President Donald Trump.
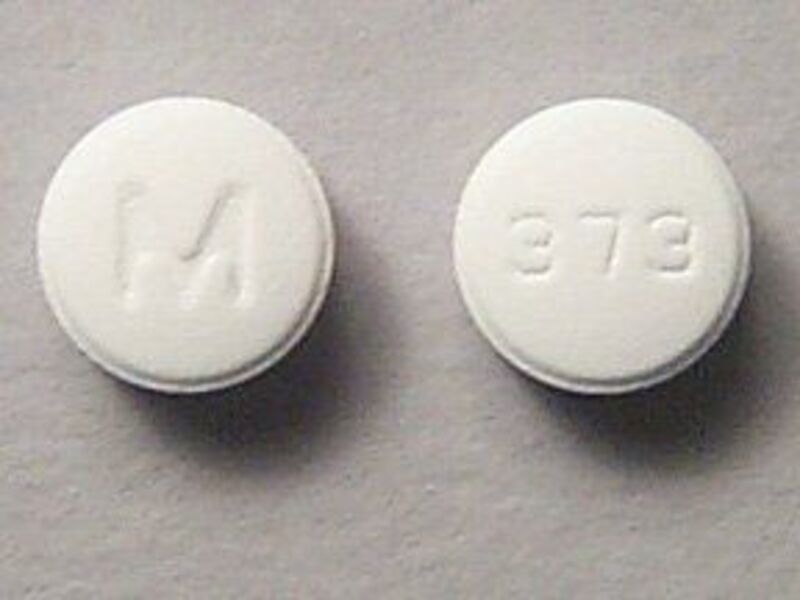
These days, Plaquenil is better known by its generic name, hydroxychloroquine. It is the medication Trump has been hyping as a potential treatment for the novel coronavirus, even though it is not approved for this use and there is scant medical evidence so far that it works to treat the virus.
Trump’s push to use hydroxychloroquine to treat COVID-19 has triggered a run on the drug. Healthy people are stocking up just in case they come down with the disease. That has left lupus patients like Valdez and those with rheumatoid arthritis suddenly confronting a lack of medication that safeguards them, and not only from the effects of those conditions. If they were required to take stronger drugs to suppress their immune systems, it could render them susceptible to more serious consequences should they get COVID-19.
The shortages have caught the attention of the Lupus Foundation of America, which said it is working “to take steps that ensure people with lupus will be protected from a disruption in access to critical medications.”
Lupus afflicts about 1.5 million Americans, and women and African Americans are disproportionately affected. The immune system of a lupus patient attacks its own tissues, causing inflammation and tissue damage in an array of organs, from the joints to the kidneys and lungs.
Many lupus patients use Plaquenil to combat these effects and have taken to Twitter with their fears for what the Trump-driven run on the drug means for them:
Valdez, 49, has been taking Plaquenil for 15 years, and it has enabled her to work and lead a relatively normal life. Here’s what she said on Twitter on Saturday, referring to Lupus as SLE, which stands for systemic lupus erythematosus.
Valdez was diagnosed with lupus when she was 31 or 32, in the early 2000s. She was working 12-hour shifts as an emergency room nurse and felt sick all the time. She chalked it up to working hard and maybe being a little overweight. “When I really started to worry is when I had a hard time gripping a soda can or managing a syringe. That’s what drove me to get seen and get a lot of testing.”
Lupus isn’t easy to diagnose, and doctors typically work to rule out other conditions first.
The first medicine Valdez’s doctor put her on was Plaquenil, a mainstay for decades that is also used as an anti-malaria drug. “Most everybody that has lupus takes Plaquenil unless they can’t tolerate it,” she said. “It’s usually the first medication that anybody with lupus is put on. It can be protective for us. It can protect our immune system from attacking our own organs. It’s the least severe of all of the medicines that are out there.”
In Valdez’s case, Plaquenil was not enough. She takes the steroid prednisone every day, as well as CellCept, an immunosuppressant drug that reduces rejection in transplant recipients and others, in addition to a weekly injection of Benlysta, which reduces her lymphocytes, the part of white blood cells that fight the immune system.
What happens if she runs out? “I am likely going to go into a flare or I am going to have to increase other, more dangerous medicines to keep me out of a flare. I take a bunch of medicines to keep my immune system from working so it doesn’t harm me. If I have to replace it [Plaquenil] by increasing my CellCept, which is a more powerful immune suppressant, I am basically putting myself at a higher risk. I am basically increasing my risk of having very serious complications of coronavirus. I already have that risk.
“When I think about the other people out there with lupus and other autoimmune disorders, we’re all really scared right now. I haven’t left my house in nine days. I’m working completely remotely. If I get coronavirus, unlike someone else my age, almost 50 years old, who is likely to recover and will be fine, I will likely end up in the ICU.”
Making Valdez even more nervous is that her 32-year-old daughter, who lives with her and has diabetes, has come down with symptoms consistent with COVID-19: fever, shortness of breath and a cough. The daughter has quarantined herself in a bedroom and uses a bathroom that no one else uses, but Valdez worries.
“She’s not here in our area,” Valdez said. “She’s not sick enough that we feel it warrants taking her to the ER to get a test. … We’re doing everything that we can but there’s no guarantee that I won’t get it.”
Valdez is angry at Trump for recommending a drug that is unproven for COVID-19, upending the way medicine has been practiced and taking a medicine that works away from her.
“When the president stands on the stage and he makes uninformed statements that are not backed by science and are not vetted by professionals who have expertise in that area, he leads an entire massive nation to think what he says is true,” she said. “You have people running around thinking there’s a cure for coronavirus, that there’s medicine.”
Some medical researchers have raised concerns about a recently published study about the use of hydroxychloroquine in coronavirus patients, and even the nation’s top infectious disease specialist has said there is no evidence, beyond anecdotes, that it works.
More robust studies are already underway. The University of Minnesota began enrolling patients in a clinical trial last week. New York State is also going to study using hydroxychloroquine in combination with the antibiotic azithromycin to treat COVID-19 patients. New York Gov. Andrew Cuomo announced Saturday that the U.S. Food and Drug Administration is acquiring 10,000 doses of azithromycin and hydroxychloroquine to be used by New York on a trial basis.
“I spoke to the president, he spoke to this drug therapy in his press conference yesterday and I spoke to him afterward,” Cuomo said Saturday. “I said that New York would be interested and we have the most number of cases and health professionals have all recommended to me that we try it, so we’ll try it.”
In light of the pandemic, some prominent hospitals, including the University of Washington, have added hydroxychloroquine to their treatment protocols. “Hydroxychloroquine is an inexpensive and generally safe drug for short term use, with few drug-drug interactions,” a university protocol says. “While it is unknown if it is effective to treat COVID-19, there is a favorable risk:benefit and cost ratio. Multiple trials are ongoing, and this recommendation will be updated when further data is available.”
As of Saturday afternoon, Valdez had 27 pills left. Now she has 25.
I asked why she didn’t just take the 10 pills the pharmacy had left.
“If I were completely out, I would have driven down there and gotten those 10,” she said. “We only take what we need, and that’s true of everything. Only take as much toilet paper as you need. Only take as much milk as you need. Or only take as much medicine as you need.”
Filed under:


Add new comment